What happens to a liquid mixture when it is driven by pressure into an initially empty container? What if the container has an ordered pattern of molecular-sized pores? To answer this question, we prepared a sort of good vodka drink – three parts water, and one ethanol – and we injected it into the pores of an hydrophobic container – the zeolite ferrierite. As hydrophobic materials don’t like water and don’t care about drinks, we had to be very drastic: we used a diamond anvil cell. In this apparatus, the sample – the empty container and the mixture, in our case – is compressed between the tips of two opposing diamonds and experiences huge pressures – about 10.000 times the normal atmospheric pressure. At these conditions, matter is subjected to unimaginable forces, comparable to internal atomic forces: this means that strange, unexpected phenomena could show up. Now, let’s combine the power of high-pressures with the ordering effect of the pore matrix and see what happens to our mixture.
Just to start with, the water-ethanol mixture – the pressure-transmitting medium – enters the pores of the matrix. But how do the molecules occupy the pores? You don’t need to be a chemist to know how it is difficult to separate alcohol from water. This is a critical issue also for sustainable processes – such as the production of biofuels.
Thanks to high pressure and to the porous matrix – and with the help of computational modeling – here we obtained the separation of ethanol from water, and the formation of a beautiful pattern of clusters. The clusters – rows of ethanol dimers, and square water tetramers – occupy different regions of the host matrix and alternate like tiles forming a nice molecular mosaic – a “two-dimensional architecture” – inside the porous host. What’s really exciting about it is that the ordered pattern, created by high pressure, also remained stable by bringing the material back to atmospheric pressure. This means that using high pressures and porous hosts, we can create new materials, which are stable at normal conditions, and could potentially be exploited in applications.
The metamorphosis of the initial water-ethanol solution into a beautiful two-dimensional pattern remains somewhat mysterious. More in general, how organization arises from chaos is still one big question in science. However, our molecular dynamics simulations show that water molecules, already inside the pores, can spontaneously self-organize in square tetramers:
The final result, is the formation of the stable two-dimensional architecture of water and ethanol clusters. As the movie shows, the molecules move, but the clusters do not break apart. – even upon returning to room pressure.
Perspectives
Disclosing the way in which molecules and nanoparticles assemble at high pressure conditions, under the guidance of a suitable matrix would be a great and intriguing challenge for future studies. Another one would be the actual production of technologically relevant materials through the combined use of pressures and suitable porous matrices. These goals could be achieved only through a close collaboration between experiment and theory – a synergy which has been at the very origin of the present work.
In a wider perspective, understanding the behavior of matter at high pressures is of central relevance in science, as explained in this excellent introductory feature article. Pressure effects are ubiquitous, in chemistry, physics, earth and planetary sciences, as well as in many industrial processes and technological applications. High-pressure conditions are also hypothesized to explain the origin of complex chemistry and life. The study of this exotic regime, so different from our everyday-life, may reveal plenty of phenomena which would be hard to imagine based on our experience.
Reference: Irreversible Conversion of a Water–Ethanol Solution into an Organized Two-Dimensional Network of Alternating Supramolecular Units in a Hydrophobic Zeolite under Pressure, by Rossella Arletti, Ettore Fois, Lara Gigli, Giovanna Vezzalini, Simona Quartieri, and myself. Angewandte Chemie 2017 – DOI: http://dx.doi.org/10.1002/anie.201610949
http://dx.doi.org/10.1002/ange.201610949
Special thanks to Andrea Stangoni (@andrea_stangoni), author of the cover artwork. His image summarizes the ideas of our work much more beautifully than my blog post!


And, of course, big thanks to Angewandte too! 🙂
Here are the official versions of the cover:
http://dx.doi.org/10.1002/anie.201700219
http://dx.doi.org/10.1002/ange.201700219
Hope you enjoyed the movies, both (equilibration and final) available at figshare.
Update: the green open access (accepted article) version of this paper is now freely downloadable from the figshare repository.
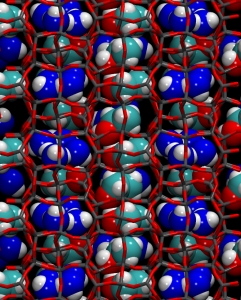
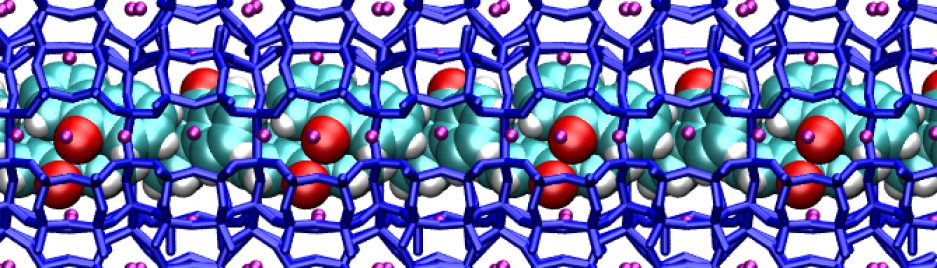
Very impressive!
Does the ethanol/water mixture behave like a superfluid or a supercritical fluid under these conditions? Could a wider zeolite (or any other framework for that matter) host not only the solvent (I’m thinking supercritical CO2) but also reagents on which to perform catalysis?
Congratulations on the cover of Angewandte!
LikeLiked by 1 person
Thank you very much for your comments and questions! The answer to the first one is no: the mixture was not supercritical, because the temperature wasn’t high enough for this mixture – experiments were performed at room temperature. instead, I think that, as you mentioned, supercritical behaviour could be possible with CO2,.
Regarding the second question, you’re totally right. Indeed, we are thinking of experimenting with larger pore zeolites and bigger molecules, and catalysis – and, more in general, reactivity – would be certainly one of the most interesting phenomena to explore at high pressure! For these developments, the experimental team would need at least some grant of time at the synchrotron.
Thank you so much again for your kind words!
LikeLiked by 1 person