The replacement of fossil fuel with sustainable alternatives free from environmental footprint is one of the most important challenges to combat climate change and meet the ever increasing energy demand of our planet. The sustainable production of hydrogen fuel through biomass-derived ethanol in Direct Ethanol Fuel Cells is a promising route, but the high costs and short lifecycle of platinum – which is still the preferred catalyst – are a serious problem. So, the quest to valid yet convenient substitutes to platinum is an open and challenging task.
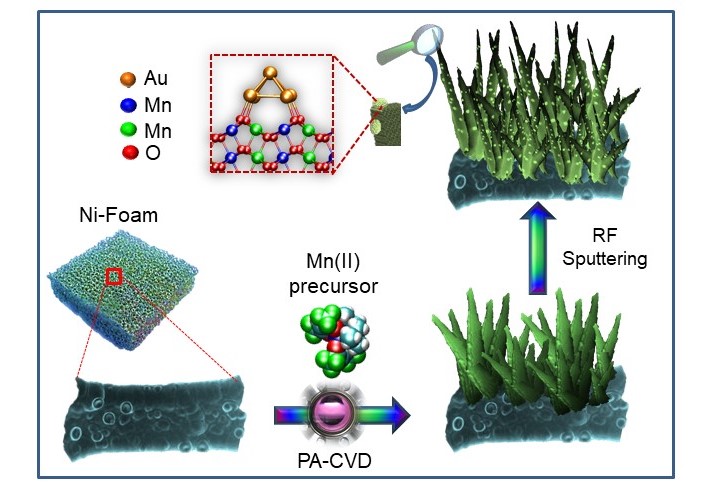
We (actually, my experimental colleagues) prepared an electrocatalysts for Ethanol Oxidation Reaction based on a low-cost and abundant metal oxide, namely manganese oxide. The fabrication strategy involves the growth of manganese oxide nanostructures on nickel foam scaffolds via plasma-assisted chemical vapor deposition and the functionalization with gold nanoparticles in low amount – as sketched in the picture below. That’s the magic of molecule-to-nanomaterials conversion!
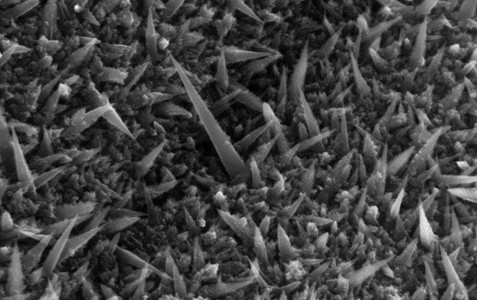
The synthesized nanostructures have large surface area and show great performances as electrocatalysts in the ethanol oxidation reaction, comparing favourably with the best oxide-based catalysts known to date. We found that a very tiny amount of gold nanoparticles is sufficient to boost the catalytic activity of manganese oxide.
Our findings not only afford a convenient route for sustainable electrocatalysts, but also explain why our catalyst is so efficient. Theoretical modeling (#compchem) showed that gold nanoparticles activate the oxide surface toward the ethanol oxidation reaction. In other words, ethanol undergoes both partial oxidation and deprotonation immediately upon adsorption on the catalyst. Hence, our catalyst optimally prepares ethanol to the electrochemical oxidation process.
This knowledge, combined with the proposed fabrication route, may guide the development of electrocatalysts based on earth-abundant metal-oxides for ethanol valorization in Direct Ethanol Fuel Cells and for (photo)electrochemical water splitting.
Personally, I enjoyed very much this work, because metal-metal oxides interfaces are particularly challenging to deal with by #compchem. Also, I like very much to interact with my experimental colleagues and friends: they always have interesting problems, and collaborating together to find a solution is often the best part of the work. Very happy that computational modeling may help to understand the complex behaviour of these intriguing materials!
We presented this work at the fabulous #RSCPoster conference 2021. Here’s a pdf copy of our poster.
This work was published in J. Mater. Chem. A, 2020,8, 16902. A free author version is available on Figshare