Chemical warfare agents put at stake human life and global safety. These compounds are extremely toxic, and their efficient detection is crucial.
By combining experiments and theory, we realized a new sensor, based on manganese oxide and gold nanoparticles, which has ultra-low detection limits and impressive selectivity towards an important simulant of the vesicant nitrogen mustard gas.
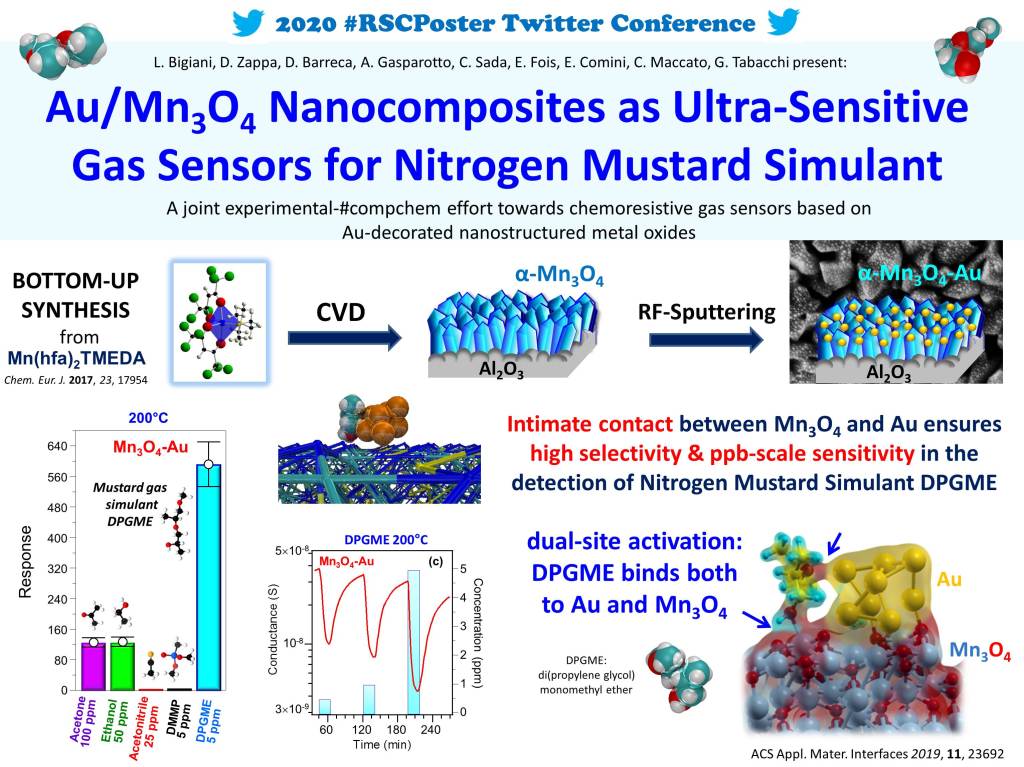
The manganese oxide nanomaterials were synthesized by chemical vapor-deposition (CVD), starting from a Mn(II) molecular complex. This compound can be easily vaporized, and gives manganese-oxide materials of high purity. Then, the manganese oxide surface was partially covered by gold nanoparticles.
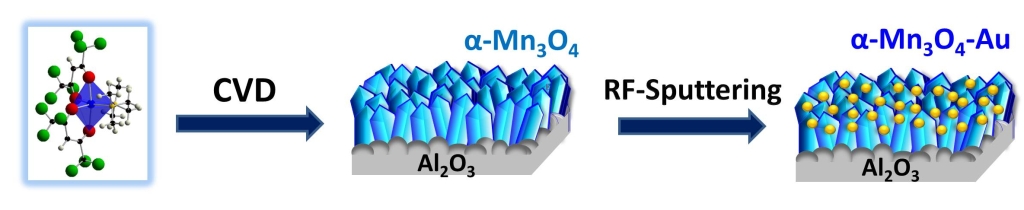
The material was then tested in the detection of a nitrogen mustard gas simulant (named dipropylene glycol monomethyl ether, DPGME). The results were exciting: the sensor showed high efficiency and selectivity, with a detection limit of 0.6 ppb.
All this work was done by our awesome experimental colleagues. However, I want to show you that also theoretical modeling (#compchem) has done its part here, by answering the question: how does this system work?
At molecular level, the sensing action depends on the contact of the analyte molecules with the active part of the sensor – the gold/manganese oxide layer. By using a density functional approach, we have seen that the molecule strongly binds to both gold and metal oxide, as shown in the picture below.
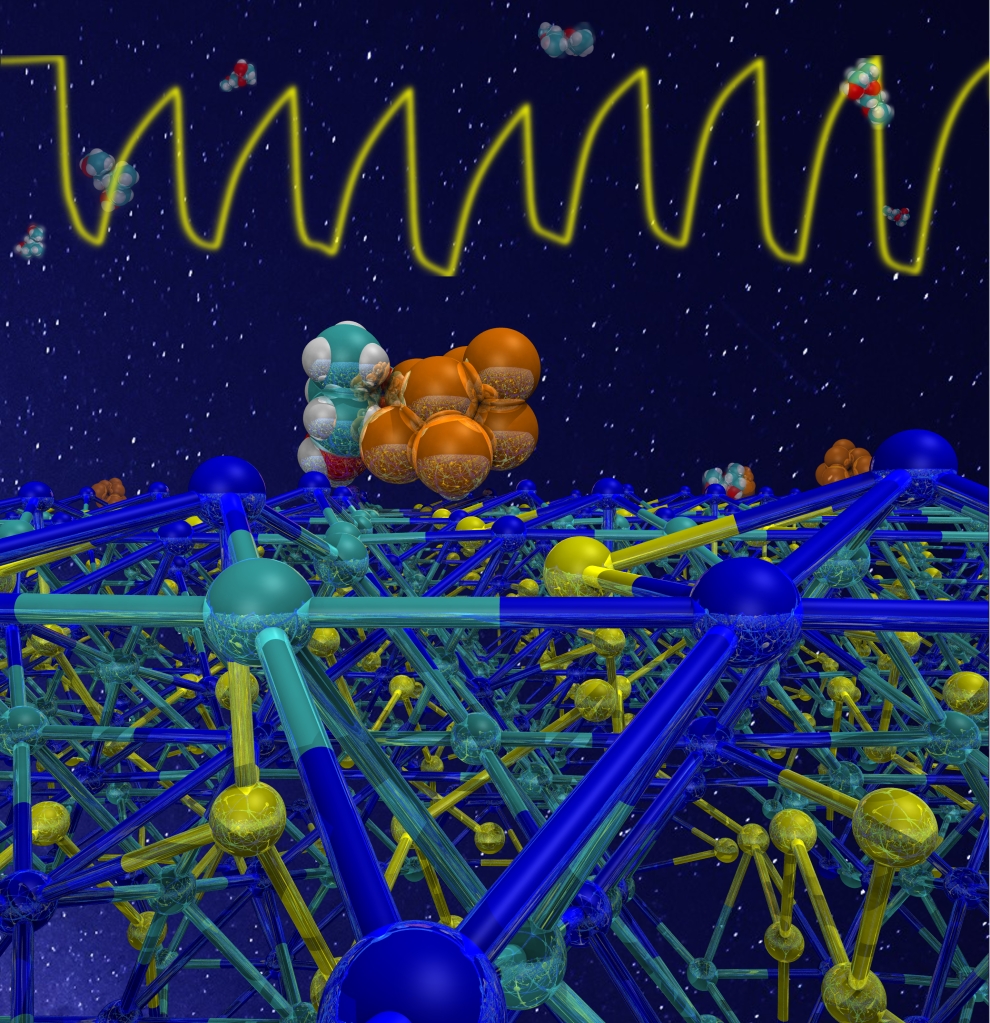
To check if this “molecular recognition” ability of the sensor was specific only to the target molecule, we modeled also the contact of ethanol with the sensor.
We found that while the mustard gas simulant is in intimate contact with both gold and Mn3O4, ethanol interacts only with the oxide surface, but not with gold. This can explain the higher response and selectivity of DPGME with respect to ethanol.
In short, the new materials have a low fabrication cost and remarkable sensing capabilities. The reason of their impressive performances in the detection of the mustard gas simulant is that the target molecule is anchored to both manganese oxide and gold.
I was really proud to present this work at the #RSCPoster2020 event – and I thank the organizers for this fantastic (and fun) opportunity.
By the way, if you still don’t know what a #RSCPoster conference is, follow #RSCPoster on Twitter, have a look to the many many great posters presented this year, and consider taking part to the 2021 edition!
Here’s our little contribution to this wonderful global event
If you’d like more information this work – including the technical details of both modeling and experiments – these can be found in our published paper (see here for a free green open access version).