Wearing sunscreen everyday decreases your risk of skin cancers and helps your skin maintain a healthier look. But how does a sunscreen work? The chemistry of a sunscreen lotion, nicely pictured in this infographics, is actually quite a complex matter. The key ingredients are molecules known as UV-filters, which can absorb UV light and then dissipate the energy harmlessly in the form of heat.
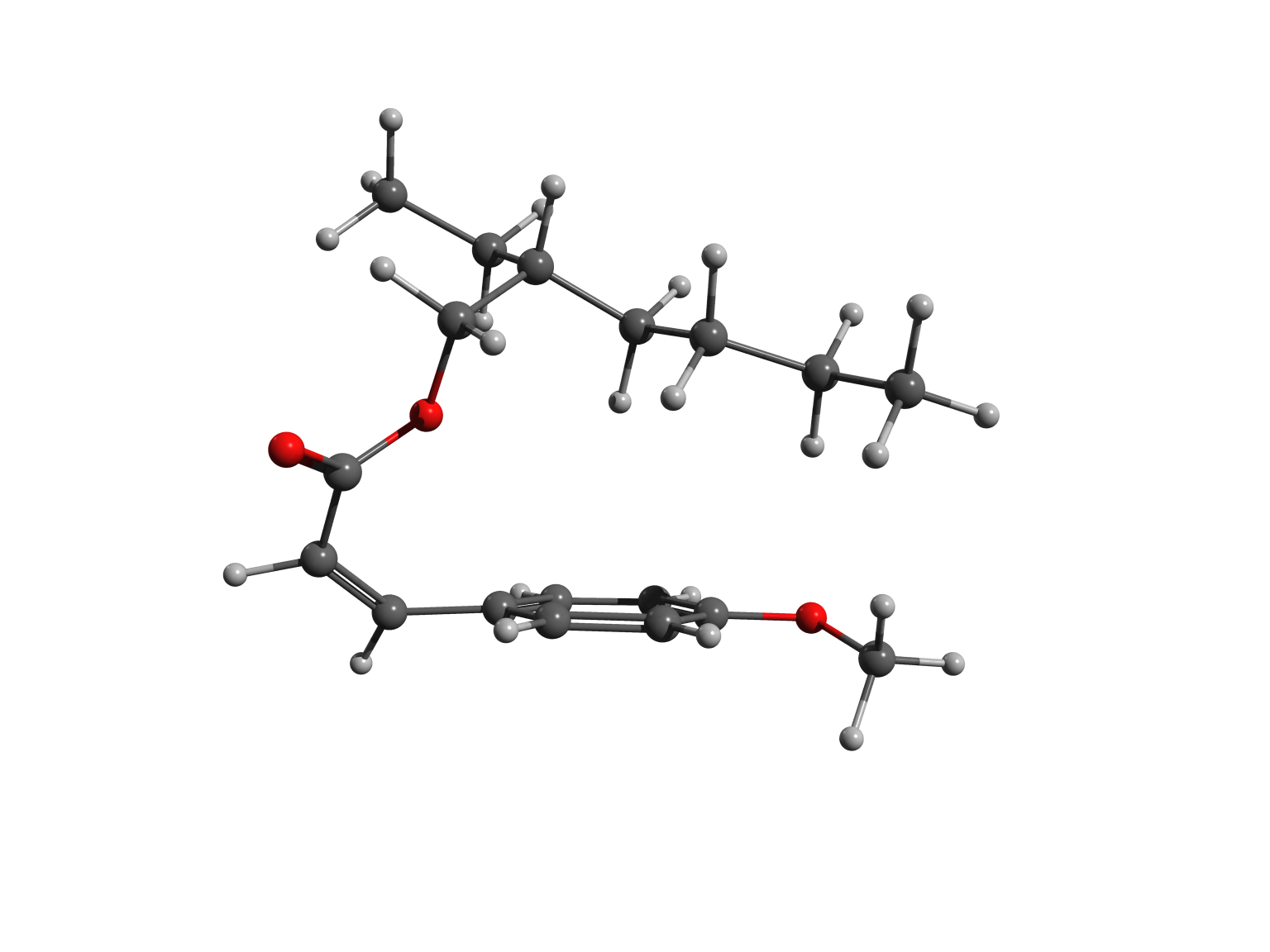
Octinoxate is one such molecules. The active part contains a ring system and conjugated double bonds. It has a flat structure – denoted as “trans“- and excellent UV-B filter properties. Unfortunately, its filtering action is degraded by light over time. Under sunlight, the molecule is converted to a less effective form, named “cis“. Understanding why this process occurs might help to improve the efficacy of sunscreen lotion and creams.
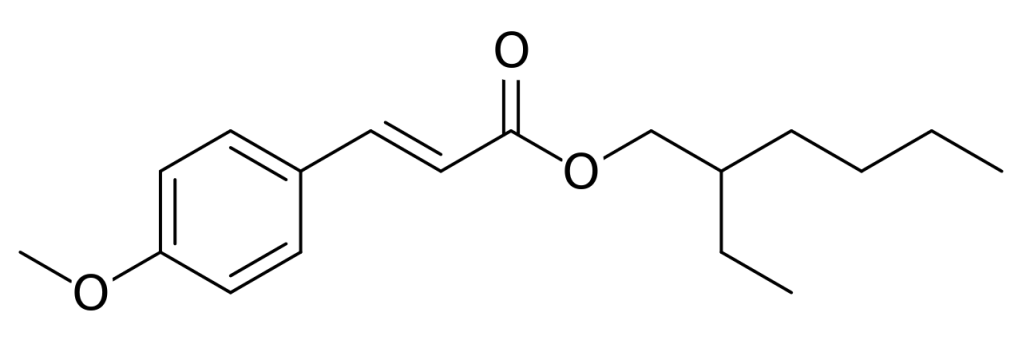
We studied with accurate calculations the two forms of the octinoxate molecule. In contrast to what was previously thought, the less active cis form is slightly more stable than the commercially valuable trans form. This finding explains why the UV filter loses efficacy, and may suggest possible ways to limit the degradation processes.
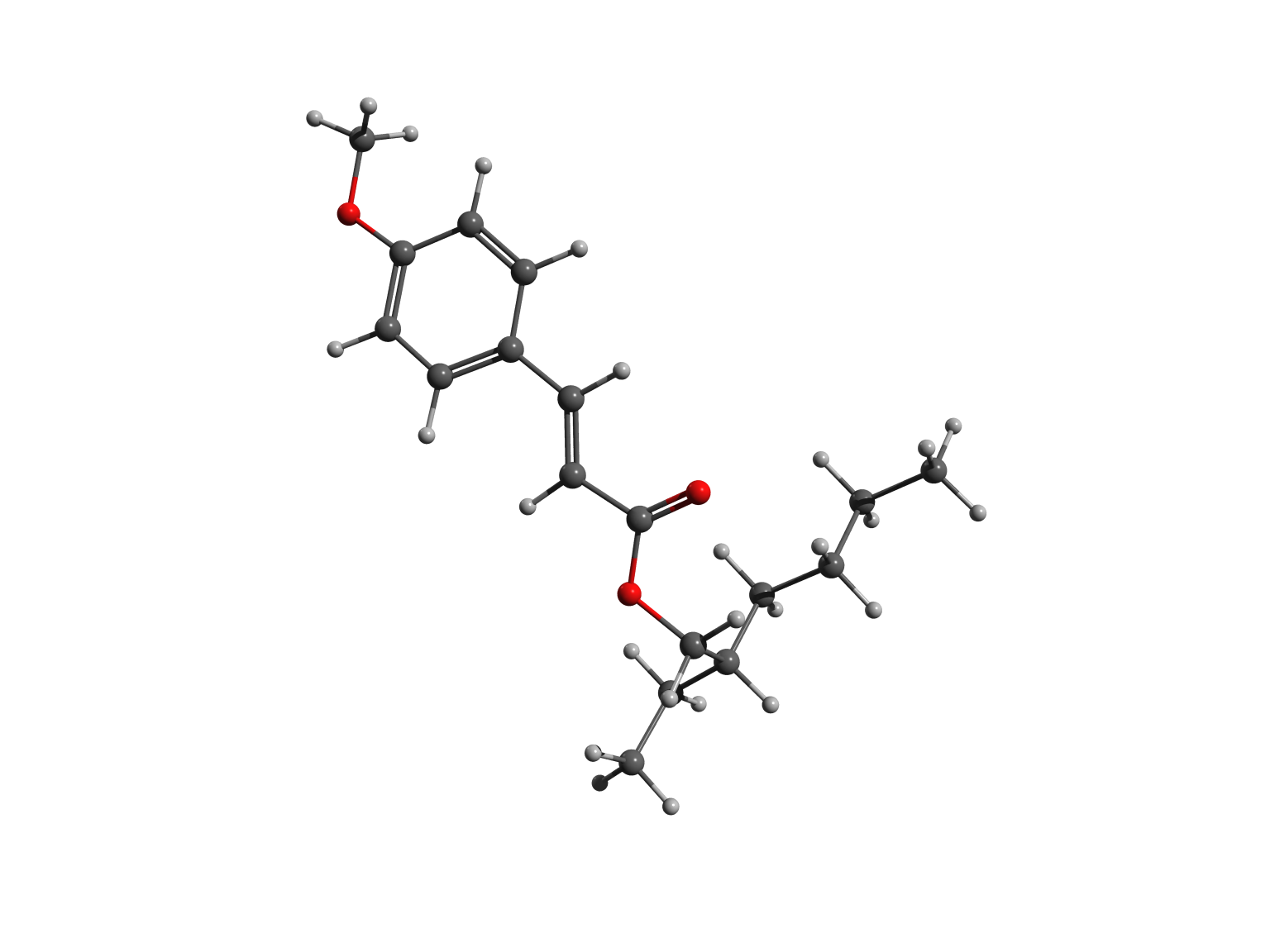
The two forms – or isomers – of the molecules have very different shapes. The trans is approximately flat, as shown in the left picture, while the cis is bent, as illustrated in the right image.
In cis octinoxate, the alkyl chain folds over the other part of the molecule, leading to a more compact shape, stabilized by dispersion interactions. Practically, the chain plays a key role, because it allows the filter to be easily dispersed in the lotions and creams we commonly use to protect our skin.
More technically, these subtle effects, highlighted by post-Hartree Fock calculations, are not fully captured by density functional approaches. All the tested functionals, including the B2PLYP double hybrid, predict the trans structure to be more stable than the cis one. This molecule is indeed a challenging system for any computational approach.
The striking difference of shape between nearly flat trans-octinoxate and folded cis-octinoxate suggests that this change of shape might help to dissipate the energy accumulated upon light absorption. Perhaps, this effect might also be beneficial to the sunscreen action.
As a further step to understand the peculiarity of this molecule, the cis– and trans- forms of octinoxate could be also investigated via molecular dynamics simulations to better capture the differences in their complex behaviour.
Links to the article , the free accepted manuscript and preprint version.