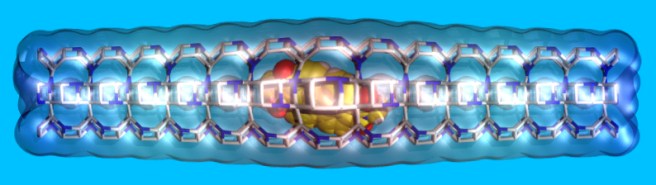
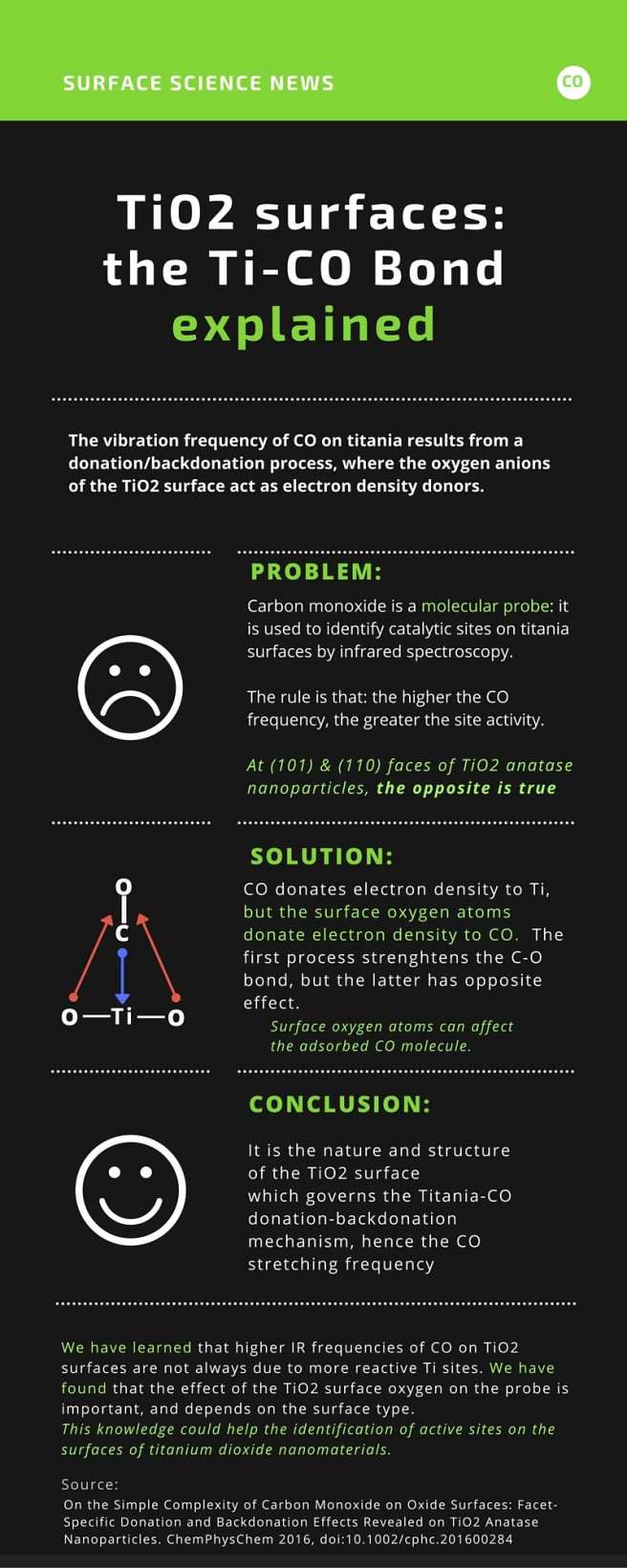
il diavolo fa le pentole ma non i coperchi
The title of this post is the literal translation of a proverb. The proverb means that Devil’s pot of wickedness sooner or later will boil – and, as there’s no lid, someone will see its content and reveal the truth. That’s the old innocent idea that, finally, justice will prevail over evil… well, I like it so much I use it as title. Rather than devils, this post is actually about pots and lids – of molecular size, of course.
As that’s not a Masterchef contest at the nanoscale, let’s get rid of the pot for the moment, and call it ‘container’. In the nanoworld there are many such containers, which can be filled with molecules. In this way, you can produce new materials with applications in various areas of technology: from solar energy to sustainability and human health.
Our containers are named zeolites – porous materials which are commonly used as adsorbents and catalysts in various commercial, industrial, and even medical applications as well as in our everyday life. Also, if you fill zeolites with dye molecules, you’ll get materials able to capture and transfer solar energy very efficiently. You would do it much easier if you first know how their pores look like.
In particular, how do their entrances appear to an incoming molecule? This question is our “step one”, because this information is really hard to get from experiments.

Fortunately, modeling comes to the rescue…. and that’s one of the reasons why I love so much doing #compchem (computational chemistry)!!

Step 2 revealed that the channel openings expose hydroxyl groups, and look somewhat like this:

Those terminal hydroxils can be condensed with other molecules, carrying specific groups, hence new properties and functionalities. Among them, the possibility of “closing” the pores. Why is it so important?
Zeolites are resistant to heat and pressure, and act as a protective shield around the dye. But every “pot” needs a “lid”: plugging the zeolite pore entrances, so that the dyes, once included, cannot escape into the environment, would further enhance their stability. This has already been done experimentally, by attaching at the channel entrances peculiar molecules nicknamed “stopcocks”. They consist of two “parts”:
- the “tail”, which can penetrate zeolite pores;
- the “head”, which is too big to enter the pore and remains outside, thus blocking (at least partially) the channel opening.
Two typical stopcocks, one with a small tail, and the other with a long, bulkier tail, are shown below.

Such “molecular stoppers” do indeed a great job in preventing molecules to escape from zeolites. However, there were no clear ideas about how these stoppers were attached to the pore entrance, and how much space they occupied. This knowledge would help finding better “lids” for our zeolite “pots”. How do we get it? Of course by modeling, as sketched in step 3 and 4.
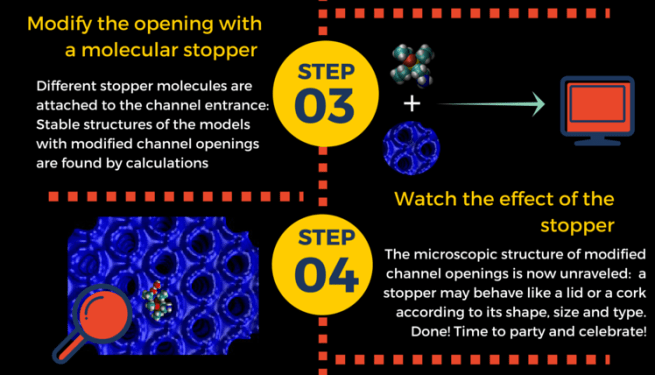
Here’s what we learned:
-
stopper molecules prefer to bind aluminum sites at the channel entrance;
- the tail group always penetrates inside the pore, while the head stays outside;
-
the extent of blocking depends on the stopcock.In particular:
– small-tailed stopcocks are like partially opened “lids” : no full closure – bulky-tailed stopcoks behave like “corks”: full closure
So the zeolite pore may be fully sealed by one bulky stopper, like a molecular cork on a Prosecco nano-bottle. On the contrary, one “lid” (small stopper) leaves our “pot” partially opened. Fortunately, there’s enough room to attach a second small stopper to the opening, that can now fully be closed.
And this brings us to step 5…

… which could well be the end of this story, first told some time ago. Thank you for reading it!
Anyway, there’s an epilogue, which is perhaps the nicest part (“dulcis in fundo“). Using such information, obtained from modeling, experimental colleagues recently trapped indigo (that’s, your denim’s blue) in zeolite L, and blocked the channel entrances with two small stopcocks. In this way, they made a new pigment, exceptionally resistant, with an amazingly beautiful blue color. For me #compchemist, that blue was simply….. the color of happiness.

For more information…
- Free to read: i) the green open access version of our article, “Structure of Nanochannel Entrances in Stopcock‐Functionalized Zeolite L Composites“; ii) a conference paper (“Chemistry at the entrance of zeolite nanochannels“); iii) a poster. For a general introduction to dye-zeolite L materials, see this open-access review.
- Requiring subscription: i) Indigo in the nanochannels of zeolite L (Woodtli et al, Dyes and Pigments, 2018, 149, 456); ii) Invention of the stopcock (Maas & Calzaferri, Angew Chem 2002, 41, 2284); iii) Official version of our article: Structure of Nanochannel Entrances in Stopcock‐Functionalized Zeolite L Composites, Angewande 2015, 54, 11112