Overwhelmed with the increasing flow of new scientific discoveries and related literature? You’re not alone. We live in the information overload era: too much to read, too little time, and life is short. Probably we’d need more readable, shorter papers too. Why writing a long one? Perhaps, it might connect disciplines which speak different languages but have much in common. Like material science and mineral science.
Let’s start from the first one.
You can make materials for solar cells, optical devices or medical sensors by trapping molecules or nanoparticles inside a “host”. Once there, molecules are no longer free to move, like in a gas or a liquid. This process, called “confinement”, brings to life new properties, which were not present in the individual molecules and are very useful in technology. Energy transfer or information storage, for instance, are made possible by the organization of the confined molecules.
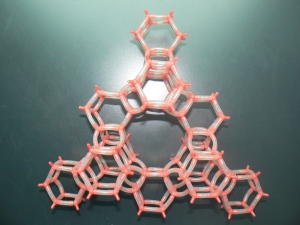
Tiny smart objects such as molecular machines, motors and diodes, make good use of self-organization processes, which create order from apparent disorder by exploiting interactions between molecules. This task gets easier when molecules are confined in regular pores. Think of a buzzing swarm of bees, first frantically hovering in the air, and then accommodated in a honeycomb.
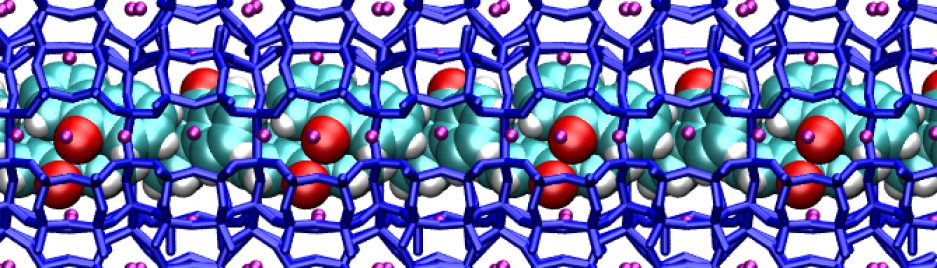
Similar to honeycombs, regular patterns of pores like those in zeolites can orderly accomodate small molecules or clusters. But if you want to entrap, say, enzymes, peptides, or large nanoparticles, you must use materials with larger pores. Some porous silicas have large honeycomb channels, while the cavities of metal organic frameworks display an amazing variety of size and shape. With those nice architectures awaiting to be filled, ordering molecules might appear like an easy task.
As you imagine, things are more complex. Perfect order cannot be achieved. All cavities would need to be uniformly occupied by the guests. This is going to be very unlikely, because molecules move a lot even when they’re confined… like bees in a hive.
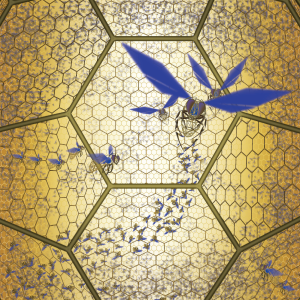
About bees, I had direct experience… as a child, I used to observe my dad opening up his hives to inspect them. This gave me the chance to “study” the behaviour of these awesome creatures inside their honeycomb.
Bees do not occupy all hexagonal holes in the frame, and move continuously around, without any apparent pattern. Hence they’re not perfectly ordered. In spite of this, the colony is amazingly organized, and performs an impressive number of complex tasks…. not just honey production!
Similarly, guest molecules confined in porous cages are not rigorously ordered. Yet they are organized, and the resulting host-guest materials can perform useful functions, which were absent in the free molecules. They can, for example, absorb and transfer photons like the antenna systems of plants and bacteria.
Now, the question is: can we improve the organization of the molecules and the performances of the materials? Well, first we should know how the molecules occupy the cavities, their orientation, spacing and so on. Are the guests aligned? Are they attached to the pore walls? What happens if water enters the pores? To find those answers, you should use several different techniques: each experiment will give you some pieces to compose the puzzle. And yes, computational chemistry helps a lot to figure our what happens inside the pores. Yet this remains a very difficult problem.
This is where mineral science might help.
Regular patterns of cages are very common in the mineral world. Not long ago, for example, geologists found in Antartica a mineral with the same structure of zeolite Z-SM5, a well-known and widely used artificial industrial catalyst. That was indeed a big surprise! Natural zeolites are indeed amazing: their pores contain impressively stable structures formed by small molecules and cations. Just look at this water wire:
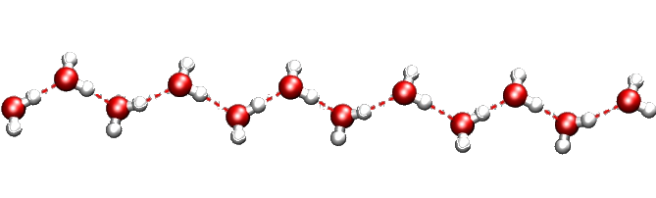
Contrary to what you’d expect, this chain is incredibly resistant to heat and pressure. First found in a rare mineral, it was named “one-dimensional ice”. But actually, our water wire “melts” at about 340 C inside the mineral framework! This is a great example of organized structure made by Nature. You can find many others: the most famous ones are perhaps gas hydrates. Several silica minerals have hydrate structures, which are also very common in man-made porous materials. Indeed, we should pay more attention to the close links between natural and artificial host-guest materials.
Natural porous minerals, the intriguing organization of their guests, and their response to mechanical stress can be an awesome source of inspiration in the quest of more robust and efficient materials. High pressure experiments with zeolites (and also some MOF’s) have already brought us new organized materials, along with many curious facts. But there’s so much yet to be discovered.
Perhaps, the problem with us (me included) and with our scientific era is that we don’t take enough time to relate with other disciplines. I’ve been so lucky to work with many awesome colleagues from the mineral, chemical and material science communities over the years, and it’s thanks to them that I wrote this review. One thing I learnt is that we should always try building bridges and strenghtening links between different fields because there’s nothing to lose, all to gain from a deeper exchange of ideas.
For more information….
- Official version of record: Supramolecular organization in confined nanospaces, ChemPhysChem 2018
- Preprint version of the review, free to download
- “free to read” link to the paper: https://rdcu.be/No7p (but you can’t download it or print it) – thanks to @fxcoudert for letting me know of it.
- Database of zeolite structures of the International Zeolite Association (IZA-SC)
- The Fascination of Crystals and Symmetry, a blog with a beautiful collection of mineral structures and other useful information on crystals
Reblogged this on The Fascination of Crystals and Symmetry and commented:
Thoghtful and very well written. Nice analogy with the bees!
LikeLiked by 1 person
Thank you so much for your comment! I am very glad you liked the analogy: it seemed to me the easiest way to explain the theme of this review. It was very kind of you to reblog this post: thanks a lot!
LikeLiked by 1 person